Give any two uses of pH in everyday life other than mentioned in the context.
In the diagram given below when electricity is passed through an aqueous solution of a common salt, A substance ‘Z’ is produced along with the evolution of gases ‘X’ and ‘Y’. When a burning matchstick is brought near the gas ‘Y’ it burns with a pop sound, whereas X is used for disinfecting drinking water. When gas ‘X’ is passed through a solution of slaked lime, an insoluble substance ‘A’ is produced.
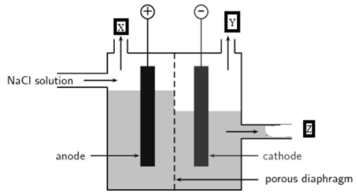
(a) Write the name of gases ‘X’ and ‘Y’.
(b) Write the balanced chemical equation for the formation of substance ‘A’.
(c) Write your observations:
(i) if a drop of blue litmus solution is added to the aqueous solution of substance ‘Z’
(ii) if methyl orange is added to substance ‘Z’